
CATER Mask
Decisions
February 17, 2021
Markets
for Air Filters, Controls and
Masks Changing Rapidly
Experts Call on Biden and OSHA
to Require Masks With the New
Level 2 80% Efficiency Rating
and for a CDC Consumer Mandate
as Well
New ASTM Standards to be
Discussed on March 2 INDA
Webinar
Freudenberg Receives FDA
Clearance for Surgical Masks
Lydall Urges Consumers to Buy
ASTM-Certified and Buy
American-Made
________________________________________________________________________
Markets for Air Filters,
Controls and Masks Changing
Rapidly
New ASTM mask standards
published this week will very
likely result in the demise of a
$15
billion U.S. market for
cloth masks and help create an
even larger market for masks
with efficient filter media.
The pandemic is changing the
entire market for air filters,
monitoring /controls as well as
masks.
To date the impact has
been a nationalistic trend but
long term the result will be the
opposite.
For example the U.S. will be
best served by ramping up
efficient masks capacity now and
then
subsidizing exports in
future years when the domestic
needs subside.
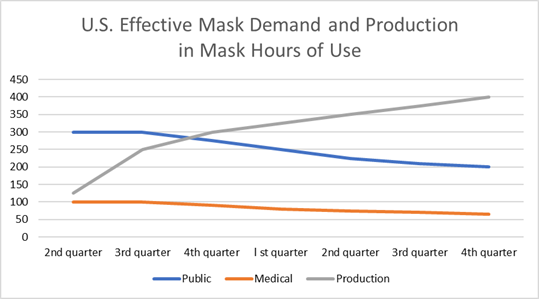
The Chinese economy is booming
due to success in minimizing the
pandemic impact. Some of the
other countries of the world
will not achieve herd immunity
for years and will be subjected
to more deadly variants of the
virus. Therefore the
markets for filters,
monitoring/controls, and masks
will continue to be
significantly impacted.
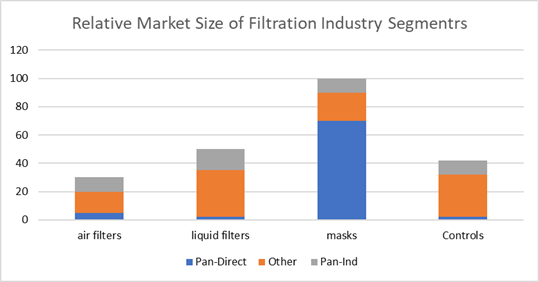
The near term opportunity for
efficient masks will greatly
exceed any other filter market.
Suppliers need to
carefully assess all the impacts
and to be proactive.
Many of the suppliers are
international. They have a
unique opportunity to help shape
the world markets.
The McIlvaine Company
with its extensive international
research and analysis
provides strategic
support for suppliers in this
fast moving market.
For more information on the
strategic support services
contact Bob McIlvaine at 847 226
2391 or
rmcilvaine@mcilvainecompany.com.
Experts Call on Biden and OSHA
to Require Masks With the New
Level 2 80% Efficiency Rating
and for a CDC Consumer Mandate
as Well
Several members of President
Joe Biden's former coronavirus advisory
board are urging his
administration to more widely
recommend and mandate the use
of N95
masks,
citing a "pressing and urgent
need for action" driven by the
threat of new coronavirus
variants.
In a memo to Biden's top
coronavirus advisers obtained by
CNN, a dozen health and safety
experts -- including four
members of Biden's former
advisory board -- called on the
US Centers for Disease Control
and Prevention and the
Occupational Safety and Health
Administration (OSHA) to
"recommend and require the use
of respiratory protection, such
as N95 FFRs (filtering facepiece
respirators), to protect all
workers at high risk of exposure
and infection."
They also urged the CDC to adopt
the first national consumer mask
standard and urged the
administration to "coordinate a
national effort" to distribute
National Institute for
Occupational Safety and
Health-certified respirators and
ASTM barrier face coverings to
workers in need and use the
Defense Production Act to ramp
up mask production.
The memo, which is addressed to
Biden's coronavirus czar Jeff
Zients, CDC Director Dr.
Rochelle Walensky and Dr.
Anthony Fauci, came just days
after the CDC updated its
guidance on face masks. The new
guidance for the first time
heralded the benefits of
double-masking but maintained a
recommendation against the use
of N95 masks "in non-healthcare
settings," citing a shortage of
N95s.
But the memo's signatories --
including former Biden advisers
Rick Bright, Dr. Celine Gounder,
Dr. David Michaels and Michael
Osterholm -- say there are now
millions of N95 masks "available
and sitting in warehouses," and
are urging the administration to
go further.
"While COVID-19 infections and
deaths have started to decline
in recent weeks, they remain at
a very high level and, unless
strengthened precautionary
measures are implemented, the
new variants will likely bring
an explosion in new infections,"
the experts write in the memo.
"Action is needed to better
protect workers and the public
against inhalation exposure to
the virus."
Bright, Gounder, Michaels and
Osterholm were part of Biden's
16-member coronavirus advisory
board, which Biden assembled
during the transition to advise
him on the pandemic and help him
craft a response strategy. The
board was dissolved once Biden
was inaugurated and its three
co-chairs joined the
administration.
Bright previously headed the
Department of Health and Human
Services' biotechnology research
arm before becoming a
whistleblower in the early
months of the Trump
administration's coronavirus
response. Michaels is an
epidemiologist who headed OSHA
in the Obama administration.
Gounder and Michaels are both
infectious disease experts.
The former Biden advisers were
joined in writing the memo by
leading public health and
occupational safety experts,
including Lisa Brosseau, Dr.
Lynn Goldman, Dr. Yoshihiro
Kawaoka, Linsey Marr, Dr. Donald
Milton, Kimberly Prather, Dr.
Robert Schooley and Peg
Seminario.
In a statement responding to the
letter, the CDC touted the
effectiveness of "properly worn
... well-fitting cloth masks"
and pointed to a CDC study
showing that "exposure to
aerosol particles was reduced by
more than 95%" when
double-masking or wearing a
tightly-fitting surgical mask.
"For reasons supported by
science, comfort, costs, and
practicality, CDC does not
recommend the use of N95
respirators for protection
against COVID-19 by the general
public," the CDC said in a
statement, though it did not
address the question of
recommending N95 masks to
high-risk workers.
The memo follows a similar
letter from some Democratic
lawmakers sent on February 1,
which called on Biden to
increase the supply and
availability of higher quality
masks and encourage the
education of the public on which
masks are most effective.
The latest memo urges the
administration to follow the
example of several European
countries like Germany and
France, which have mandated the
use of N95-style masks and
higher-quality masks in
workplaces and public places.
The authors also called on the
CDC to adopt the first national
consumer mask standard to
protect American workers and
general public established by
ASTM, an international technical
standards organization. That
standard outlines minimum fit,
design, performance and testing
requirements for consumer face
masks and will soon allow the
public to be able to choose
between two levels of mask
protection seen on package
labeling.
The letter also asks OSHA to
utilize the ASTM standard and
offer high-performing face masks
with at least 80% filtration
efficacy to non-health care
workers at lower exposure risks.
While the memo's authors praised
the administration's coronavirus
response plan, the memo is one
of the first signs of outside
pressure urging the White House
to do more amid the threat of
new, more transmissible
coronavirus variants.
In the memo, the experts urge
the administration to rescind
recommendations advising
healthcare workers not involved
in direct patient care and
high-risk workers like those in
meat plants to only wear face
coverings or surgical masks --
rather than N95s.
In a nod to Biden's focus on
equity in his coronavirus
response, the authors also noted
that the risks of transmission
are especially acute for people
of color.
"The failure to address
inhalation exposure to
SARS-CoV-2 continues to put
workers and the public at
serious risk of infection,
particularly people of color,
many of whom work on the front
lines in essential jobs and have
suffered -- and continue to
suffer -- the greatest impacts
of the COVID-19 pandemic," the
memo says.
New ASTM Standards to be
Discussed in March 2 INDA
Webinar
INDA, the Association of the
Nonwoven Fabrics Industry,
announced a webinar date of
Tuesday, March 2, at 11 a.m. EST
to discuss the new ASTM F3502-21
Standard Specification for
Barrier Face Coverings launched
by ASTM on February 15.
This standard establishes for
the first time a set of test
methods that evaluate the
filter, fit and leakage
performance of barrier face
coverings, commonly referred to
as “face masks” worn by the
general public, and not to be
confused with respirators nor
medical or surgical masks. The
webinar will be presented by
respiratory expert, Jeff Stull,
vice chair of the ASTM Committee
that wrote the standard, and
Dave Rousse, INDA president.
It will provide a detailed
review of the new ASTM standard
and the test methods it entails
and the impact on the entire
supply chain of facemask
production. Details and
registration are available here:
www.inda.org/inda-webinars
The purpose of guidance for the
general public to wear face
masks is to control the spread
of viruses. Face masks made to
this new ASTM standard will also
provide a degree of particulate
filtration to reduce the amount
of inhaled particulate matter.
The goal of the standard is to
assist consumers in making
informed decisions about face
masks given the vast array of
products currently for sale,
including various patterns
promoted for homemade
manufacture using common textile
materials. Prior to the ASTM
standard, no standard test
method existed which allowed
comparisons among different
products nor were there any
minimum performance
requirements. This new standard
provides these performance
requirements as well as a set of
specifications, guidelines and
expectations for face mask
manufacturers and media
suppliers.
“We approached NIOSH last year
on developing a general public
face mask standard that could
use nonwoven materials beyond
meltblown that still deliver an
effective level of filtration,
as there was so much demand for
the N95 respirators and masks
once the Asian supply chain was
cut off,” said Dave Rousse, INDA
president. “We were delighted to
get a positive response from Jon
Szalajda, NIOSH deputy director,
National Personal Protective
Technology Laboratory, who is
also the chair of the ASTM
Committee dealing with standards
in this area.”
“This was a worthwhile project
that we worked through the ASTM
process in record time,”
Szalajda said. “It should
provide an important benefit in
the fight against COVID-19
spread by reducing consumer
confusion about what works and
what does not and assisting
manufacturers in making
effective products.”
“The development of this
standard has been followed by
the Occupational Safety & Health
Administration (OSHA),” Rousse
said, “as it considers the
January 21 Presidential
Executive Order on whether any
emergency temporary standards
with respect to masks in the
workplace are necessary to
reduce the risk of COVID-19
spread, and if so, to issue them
by March 15. This would have a
significant and rapid impact on
the facemask industry. In our
March 2 webinar, we will review
all of these developments and
provide needed information and
guidance to the suppliers,
converters and marketers in this
important sector.”
This webinar on March 2 will be
in addition to the INDA Webinar
Series already scheduled for
March 16, March 25 and April 6.
For information on the full
webinar series, visit
www.inda.org/inda-webinars.
Freudenberg Receives FDA
Clearance for Surgical Masks
ASTM Level 3 surgical masks
manufactured by Freudenberg
Performance Materials recently
received 510(k) clearance from
the US Food and Drug
Administration (FDA). The
surgical masks are intended for
use by healthcare personnel to
protect both the patient and
them from transfer of
microorganisms, body fluids and
particulate material. The
face masks are intended for use
in infection control practices
to reduce the potential exposure
to blood and body fluids.
For the U.S. market, Freudenberg
Performance Materials is now
providing FDA cleared surgical
masks meeting the Level 3
standard of the American Society
of Testing and Materials (ASTM).
The ASTM Level 3 surgical mask
is for use in conditions where
there is a high risk of fluid
and spray of aerosol
transmission, such as operating
procedures. The masks are a
single use, disposable device
provided non-sterile.
Surgical masks by Freudenberg
were tested for performance in
five areas: fluid resistance,
differential pressure,
particulate efficiency,
bacterial filtration efficiency
and flammability. Upon
completion of testing, the
surgical masks consistently met
ASTM Level 3 criteria in all
five performance test areas.
Bacterial and particle
filtration efficiency test
results were greater than 99%.
“This was a great accomplishment
for our team. It is a milestone
that supports our commitment to
continue manufacturing local.
Meeting the ASTM Level 3
standard helps us in our goal to
not only keep our healthcare
workers safe, but also ensure
they have a steady, reliable PPE
supply so the shortages that
happened at the beginning of the
pandemic don’t happen again,”
says Alicen Pittenger, Head of
Sales – North America – Global
Healthcare Division, Freudenberg.
The Durham, NC site of
Freudenberg Performance
Materials began mask production
in 2020 in response to the
COVID-19 pandemic. Working
closely with local partners,
Freudenberg is also aiming to
prevent future PPE supply
shortages by establishing
long-term face mask production
for the US market. The site
manufactures surgical masks and
community masks and is pursuing
NIOSH N95 respirator
certification.
Lydall Urges Consumers to Buy
ASTM-Certified and Buy
American-Made
ASTM International has
introduced new certification
standards for General Barrier
Face Coverings, giving the
general public greater awareness
and control over the level of
protection provided by cloth
face masks. ASTM Level 1 Face
Coverings block at least 20% of
particles 0.3 micron and larger
(including bacteria and many
viruses), while ASTM Level 2
Face Coverings block at least
50% of these particles.
In response to these new
standards, Sara Greenstein,
President & CEO of Lydall,
Inc.,
provided the following
statement:
“Lydall
and our team of filtration and
materials science experts were
honored to work alongside ASTM
International, the CDC, INDA and
our fellow industry colleagues
in this joint effort to better
educate consumers about the
level of protection face masks
provide against threats like
COVID-19. Since the onset of the
pandemic, manufacturers all over
the world have stepped up to
accelerate production of
personal protective equipment
and the materials they require
but demand still heavily
outweighs supply. For that
reason, it remains critically
important that we right-size our
face masks for our level of risk
– and in order to do that, we
must understand exactly how much
protection masks offer. N95
respirators and surgical masks
that contain fine fiber
meltblown filtration media
remain the gold standard, but
these new standards provide
much-needed guidance for a
broader range of more widely
available masks. The message is
clear: by buying ASTM-certified
and buying American-made,
consumers can have clarity and
confidence in the level of
defense their masks deliver
against the virus.”
Here are additional articles you
will find in Coronavirus
Technology Solutions
Handanhy Supplies Melt Blown
Media and Masks Worldwide
Mann + Hummel Room Air Purifier
Provides Effective Protection
From Viruses in Classrooms
Capture Virus and Dust at the
Source - Brake Filter Example
It will Take Years to Vanquish
COVID-19
