
CATER Mask
Decisions
February 10, 2021
Mask Policies Need to be Revised
NXTNano Can Contribute Nanofiber Advantages
Armbrust American is Ready to Step Up with
Substantial Production Increases
TMS Can Increase Production by 16 Million per
Month
Shortage of N95 Masks for Medical Workers Still
Exists
ASTM Standard for Respiratory Fit will Encourage
Better Designs
NIOSH September Observations on EHMPRs
SPG Tests Show That Flat Fold N95s Provided
Better Seal Than Cup Shaped
Eight Million Deaths Caused by Polluted Air Each
Year
___________________________________________________________________________
Mask Policies Need to be Revised
The initiative for OSHA was covered in the Alert
yesterday.
There is agreement that
tight fitting efficient masks will save
thousands of lives which will otherwise be lost
by people wearing cloth masks. Erick Couch is
working with INDA and McIlvaine to present
evidence which could be helpful in guiding the
Biden Administration to create appropriate
policies and funding. Facts and insights are
needed in four areas.
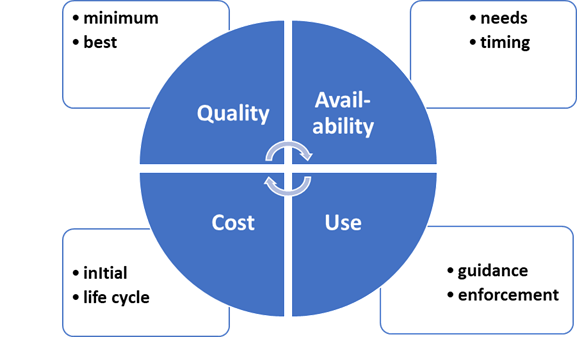
QUALITY:
Minimum standards such as being issued by
ASTM provide the first step in insuring quality.
Expert guidance will result in the best choices
using both qualitative and quantitative
methods. The role of government will be
to embrace the minimum standards and to
encourage the reliance of purchasers on expert
advice. Air leakage, breathability, and particle
removal are all important.
USE: The motivation has to be push and pull.
Push or enforcement has to be minimum standards
such as ASTM. Pull can be education as to the
advantages of effective masks but also the
creation of safe bubbles where with effective
masks more normal life can be enjoyed.
AVAILABILITY: The mask need assessment should
include uses by the medical profession,
other countries, and for other
applications as well as for the U.S. public. The
timing by which additional masks can be made
available needs to be determined.
COST: Initial mask cost and life are needed to
assess total cost. Since mask deterioration with
time may be slow and steady there is opportunity
to decrease cost and increase
availability.
NXTNano Can Contribute Nanofiber Advantages
NXTnano supplies nanofiber media for masks and
cites the life as well as efficiency as
attractive features.
Here is our interview with Andy McDowell.
Bob: What is the relationship between NXTnano
and suppliers of masks?
Andy: NXTnano works very closely with our
mask/respirator customers to down-select the
filtration media of best fit depending on their
design. Often times we will even help manage the
testing of the products through SGS or Nelson
for them, with all of our lab equipment we can
make sure their products are testing well before
going out to the labs. Once we do send them
out, we’ve looked at so many results now and can
help our customers spot pitfalls or
inconsistencies to avoid unnecessary delays. For
programs where there is N95 approval being
sought we see them as strategic partners, our
technology is effectively locked in when they
receive approval.
Bob: Since a nanofiber media mask does not rely
on a temporary electrostatic charge what is your
estimate of reusability?
Andy: We have done several studies on this
now, most recently our employees wore one of our
customers (Filti) respirators for eight hour
shifts, for five days straight. We measured the
initial efficiency, then the efficiency every
morning before they wore it again. After day one
we lost a few percentage points of efficiency,
but overall the respirators were performing very
well. After this initial loss, which was likely
inherent static, they leveled off. After five
days we called it, the respirators we getting
dirty from general handling, food, makeup etc.
but the efficiency was still over 90% on the
TSI.
Bob: Explain the range of efficiencies and
related resistance which are available.
Andy: At the moment we are most commonly
producing material to the N95 standards, in a
finished respirator form using our nano I expect
to see resistance values of 10-12 mm of H2O at
85 L/min flow with 97%-98% efficiency on the TSI
with 0.26 micron NACL (mean mass). It’s easy
enough to go down the scale and produce less
restrictive media; with something at
65-70% efficient (0.26 micron NACL) at 85 LPM
our restriction can be as low as 2.5 mm. if you
plot those two points out, establish a trend
line the fit between them and then check against
actual produced materials you will find the
“fit” is quite linear. So for a customer this
translates to a conversation of: I must have
“x" efficiency, or I must have less than “y”
pressure drop.
Bob: If an unlimited market were available how
much production could you achieve in four
months?
Andy: Our nano lines are running 24/7 right now
but we are keeping up and making running
productivity improvements weekly. When we last
talked the conversation was what would it take
for NXTNano to add more lines, we have now
pulled that trigger. Line 4 will be up in April,
and line 5 is looking like June with the option
to take it to possibly 130” wide instead of our
current 80”. The average through put we see for
customers is about 250,000 respirators / per
machine / per week on an eight hour shift. For
us that’s about 12,500 sq meters of material
they need per week per shift, which is only
around six hours of run time on one of our
lines. As we add capacity we will easily grow
with our mask customer base. For our customers,
several of them have the capability to turn out
several million masks per week based on their
current equipment, and additional equipment can
generally be added and running in 60-90 days.
Bob: What are the limiting factors in terms of
obtaining electrospinning equipment for media,
mask machines or other products and services?
Andy: Most of what people are doing in
electrospinning is at the lab scale. For serious
volume production there are only a handful (less
than 5) companies to select from globally. Most
of the equipment I’ve seen simply won’t support
economical manufacturing both from a throughput
perspective and an uptime perspective. Those
that will, are proprietary designs heavily
guarded, and rightfully so, by the companies
that operate them. Mask machines aren’t exactly
hard to get ahold of, there are domestic and
offshore options. What we have seen most
commonly is a structural issue in knowledge -
people know the mask they want to make but don’t
necessarily understand how to get home on
performance of the mask.
Armbrust American is Ready to Step Up with
Substantial Production Increases
Lloyd Armbrust
replied to the Couch request with a
forecast
of substantial production increases should
funding be available “We originally designed our
Texas facility to produce 1.4 Billion masks
annually, complete with vertical integration
making all of the nonwoven fabric in house. We
are partially through this building process
having already invested nearly $10M.
Additionally, we have real MEPCA implementation
plans, existing factory space, plus many of the
machines actually on the ground here in Texas.
“In fact, I would bet that we are the only
company in the world that has nonwoven equipment
capable of producing 300 tons per month ready
to be hooked up in less than 30 days’ time in
the US. The only issue is that our current
FDA-registered facility doesn't have the
appropriate power requirement. Because of this,
we decided to build a new facility which has
pushed our production timeline back. However, we
have all of the original plans and approvals to
move forward with our existing facility if we
wanted to pay to the premium price to bring
4000amps of power into that building.
TMS Can Increase Production by 16 Million per
Month
Dan Grayson replied to the Couch request.
He estimates that with government funding
production could be
increased by 16 million masks per month
over the next four months.
Shortage of N95 Masks for Medical Workers Still
Exists
There is a shortage of N95 mask. So Don Milton,
MD and Professor at the University of Maryland
thought it was notable that
FEMA has authorized US N95 manufacturers to
export 1 million N95 masks per month.
The main focus of the Alert today is to address
the potential with OSHA and masks for the U.S.
public. But we need a holistic approach. If
there is still a shortage of masks for medical
workers then it will be more challenging to
suppl masks to the public.
Also until the poorer countries have masks or
vaccines, the U.S. will be impacted economically
but also by the transmission of new variants.
The poorer countries also account for the
majority of the 8 million people per year dying
due to air pollutants (see article in this
Alert). So mask demand has to be viewed
holistically from a geographic and application
perspective.
A year ago, hundreds of desperate consumers were
emailing Mike Bowen's Texas medical supply
factory every day, looking to buy N95 medical
respirator masks that can filter viruses:
"Scared Americans and moms and old people and
people saying, 'Help me,' " Bowen recalls in an
interview with NPR.
Today, most consumers still aren't able to buy
N95 masks because the supply available to
retailers remains very limited. Even hospital
workers are still being asked to ration
and reuse their supplies of N95s, and the
website of the Centers for Disease Control and
Prevention says,
"N-95 respirators should not be used [by the
general public] because they should be conserved
for healthcare personnel."
Meanwhile, consumer demand for N95s and
medical-grade, surgical-style masks keeps
growing as the Biden administration emphasizes
the use of masks by the public to slow
the spread of the coronavirus — especially as
new variants of it spread rapidly around the
world.
From the start of the coronavirus pandemic,
Bowen's company, Prestige Ameritech, and most
other makers and distributors have prioritized
supplying health care workers, who say they
still don't have enough masks and other personal
protective equipment.
The Biden administration has
invoked the Defense Production Act to
prioritize production of N95s and other medical
supplies. But even with those measures, U.S.
hospitals remain worried about their supply of
these medical masks — more formally called
respirators — despite efforts by factories to
churn out billions more.
The story of N95 production over the last year
in many ways reflects shortages seen throughout
the U.S. medical supply during the pandemic —
from ventilators and exam gloves to syringes and
vaccines. The demand is global and sustained,
putting pressure on a fragile supply chain that
remains stressed and unable to keep up.
"Global demand continues to outpace production,"
says Nancy Foster, vice president of quality and
patient safety at the American Hospital
Association. Availability of N95 masks has
improved since last spring, Foster says, but "we
are continuing to use conservation measures
within hospitals to protect the supplies we
have, to extend the wear of N95s designed for
one-time use." That includes asking
hospital workers to wear each mask longer.
Costs for N95s — and other medical supplies,
like medical
gloves and gowns — have at least doubled.
The use of N95s has increased 500% since July, according
to Premier, a company that buys medical
supplies on behalf of about 40% of U.S.
hospitals.
"In most of the hospitals, nurses are wearing
their N95s for five shifts," or up to 60 hours,
says Mary Turner, president of the Minnesota
Nurses Association and an intensive care nurse
working with COVID-19 patients. "It's becoming
the norm to not wear N95s the way they're
supposed to be used."
A November
survey by National Nurses United found
the lack of protective gear like N95s remains a
huge safety concern for its members. More than
80% of nurses reported reusing single-use items
like N95 respirators, and about 20% of hospitals
had recently limited the use of N95s.
Before the pandemic, there was little consumer
demand for these products. Purchasers included
people with compromised immune systems or others
working in wildfire
areas or on dusty home improvement projects.
That has changed. Everyone — from front-line
grocery workers to travelers to teachers to
people visiting vulnerable family members — is
looking for the specialized masks.
N95s are the gold standard in masks because
unlike cloth, surgical and KN95 alternatives,
they're tested and approved by a federal agency
as having demonstrated that "they can filter out
a minimum of 95% of airborne particles under
worst case test conditions," according
to the CDC.
Nonetheless, N95s are still rarely available to
consumers.
Shepard Medical Products,
an Illinois-based company that sells supplies to
drugstores and other retailers, hasn't sold a
single N95 since March of last year. That's when
makers of N95s called the company's president,
Chris Humbert, to tell him, " 'We're done — we
won't have any more product available for 2020.'
"
So far this year, Humbert says, that shortage
hasn't yet eased. Some wholesalers large enough
to order directly from factories in China
occasionally can get N95s to sell at hardware
stores, for example, but "it's still very
fragmented." The priority, he says, has been to
supply health care facilities and government
agencies. "I stopped trying, until hospitals are
covered."
Fraud is also a major concern. Everyone, from
nurses, hospitals, manufacturers and
distributors, says vetting fake suppliers or
identifying copycat N95 masks has been a huge
concern.
Humbert says many new upstarts tried to sell him
products billed as N95s, but because he couldn't
verify their quality or efficacy, he decided it
would be safer to remain out of stock.
"We didn't like being out of stock and
disappointing any of our customers by not being
able to supply, but we did not feel that we had
a reliable source that could provide those
products for us on par with the product that we
had in place," Humbert says.
Exactly when American consumers might once again
gain broader access to N95s depends on a lot of
factors.
"I think if the vaccine rolls out faster, you're
going to be able to get N95s faster," as the
risks diminish and fewer people need N95s, says Kaitlin
Wolak,
a supply chain expert and assistant professor at
the University of Notre Dame. (Public health
workers urge even those immunized to continue
pandemic precautions — including consistent
mask-wearing — for now, until the
pandemic is tamed.)
Broader availability of N95s also depends on
manufacturing speed, Wowak notes, and on when
backlogged orders from hospitals and other
medical facilities can be filled.
The Biden administration has touted its plans to
use the Defense
Production Act to stimulate
production. Wowak says that might mean
manufacturers get more federal help finding the
raw materials needed or coordinating
distribution of supply. But it won't address
some of the main challenges that affect the
speed of manufacturing.
Wowak says how fast products like N95s are made
is determined by three primary factors: the
complexity of the equipment used to make the
product, the availability of raw materials and
the availability of trained workers.
Making vats of hand sanitizer at a rum
distillery, in other words, is very different
from ramping up an N95 factory, because of the
cost and complexity.
Managing those costs and complexities has made
the past year extremely busy for Mike Bowen, the
co-owner of Prestige Ameritech. He and his
partner started the company in 2005; it is one
of the few makers of N95s based in the United
States. Demand overwhelmed his factory a year
ago when China stopped exporting the masks that
most U.S. hospitals relied on for most of their
supply.
"I've gotten requests for maybe a billion and a
half masks if you add it up," Bowen
told NPR in late February of last
year. At the time, Bowen's company could produce
75,000 N95s a month.
He was troubled by the influx of orders, he
said. They put him in a bind.
To make more N95s, Bowen would need new mask
machines, each of which takes four months to
custom build and costs as much as $1 million. To
justify building extra machines, he needed
assurance that U.S. hospitals and government
agencies wouldn't just go back to buying cheaper
Chinese-made masks once the pandemic was over.
He'd been burned before. A decade earlier,
during the H1N1 flu pandemic, Prestige had made
what Bowen called "the mistake" of investing in
new machines and ramping up production for a
need that dried up as suddenly as it began.
"One day — and it is literally one day — it just
quits," Bowen told NPR last spring. "The demand
is over."
He eventually did decide to expand last spring,
as the COVID-19 pandemic worsened.
Bowen asked U.S. hospitals to sign multiyear
deals for N95s. That gave him the funds to build
nine new N95 machines, some of which are still
coming online. The factory now makes 80 times
more masks than it did a year ago.
"We're now selling 6 million [a month], and we
have another 4 million coming on board," he
says.
For the first time in a very long time, Bowen
says, he has some excess supply he could start
selling into the consumer market.
ASTM Standard for Respiratory Fit will Encourage
Better Designs
An evaluation of the new ASTM standard was
posted on the NIOSH website January 26,
2021 by Christopher Coffey, PhD; Lisa Brosseau,
ScD, CIH; M. E. Bonnie Rogers, DrPH; and
Jonathan Szalajda, MS.
One of the most important criteria for any
filtering facepiece air-purifying respirator to
be effective is that a good seal is formed
between the respirator’s facepiece and the
wearer’s skin. The ability to achieve this seal
is called the respirator’s fitting
characteristic.
In 1995, when NIOSH put Title 42 Code of Federal
Regulations Part 84 (42CFR84) into operation, it
did not include an evaluation of the fitting
characteristics of respirators approved only for
particulates.1 In addition, no
voluntary consensus or other government-unique
standards existed to evaluate the fit capability
of a filtering facepiece respirator prior to it
being used in the workplace in an OSHA-regulated
fit testing program. Therefore, several studies
have been conducted to determine how well
NIOSH-approved particulate respirators,
especially filtering facepiece respirators, fit
wearers.2-8 These studies found a
high number of filtering facepiece respirators
on the market at the time had poor fitting
characteristics. Filtering facepiece respirators
that do not fit most employees place an
unacceptable burden on respirator program
administrators, who must then provide many
models and sizes to ensure that every wearer can
find a respirator that fits properly.9 In
addition, poorly fitting respirators increase
the number of fit tests required, increasing
costs.10
The ASTM RFC Standard will enable respirator
manufacturers to develop better designed models
that fit the worker population. Respirators
passing the RFC Standard test method are
expected to have better fitting characteristics.
The RFC standard will lower costs to respiratory
protection programs by reducing the number of
different models needed in the program.
Purchasers of particulate-only respirators could
reference ASTM F3407 in their procurement
packages to ensure receiving those with good
fitting characteristics. The RFC Standard can be
used by organizations, such as NIOSH, to ensure
a minimum performance level of for all
respirators used within an approval program.16 Both
conventional respirator designs as well as novel
respirators (such as ones without the prevalent
two-strap head harness to provide adequate
tension during use and even distribution of
pressure) can be evaluated using the RFC
standard.19
Ultimately, this RFC Standard defines
performance requirements that could be used as
part of a conformity assessment program to
ensure that NIOSH-approved respirators will fit
a specified percentage of the intended user
population, thus providing workers with better
protection. This is crucial in all industries in
which workers are exposed to a variety of
agents, one of the most notable examples being
the Healthcare and Social Assistance industry
sector. Healthcare workers may be exposed to
biological agents, e.g., seasonal influenza,
Ebola, Severe Acute Respiratory Syndrome (SARS),
Influenza A H1N1, and more recently SARS-CoV-2,
the virus that causes COVID-19, as well as to
chemical agents.17-19
ASTM F3407 can be read for free at https://www.astm.org/COVID-19/ or
purchased at https://www.astm.org/search/fullsite-search.html?query=Respirator%20fit%20capability.
NIOSH September Observations on EHMPRs
The high demand and limited supply of N95
filtering facepiece respirators (FFRs) during
the COVID-19 pandemic have led organizations to
rely on other types of respirators, such as
reusable elastomeric half mask respirators (EHMRs).
Photo Courtesy of MSA
An EHMR is a non-powered NIOSH-approved
respirator that has a tight-fitting facepiece
that covers the nose and mouth.1 The
facepieces are made of synthetic or natural
rubber material permitting repeated cleaning,
disinfection, storage, and reuse. EHMRs use
replaceable filters or cartridges, and they
provide the same, or greater, level of
protection as single-use N95 FFRs. While EHMRs
have been used in many workplace settings for
years, their utility in healthcare settings has
not been as common.
CDC developed strategies to optimize the supply
of EHMRs during conventional and surge demand
situations, as experienced during the COVID-19
pandemic.1 NIOSH-approved EHMRs
provide an alternative respiratory protection
option capable of reducing the total number of
respirators required because EHMRs may be
cleaned, disinfected, and reused numerous times.1 Unless
the EHMR filter cartridges become visibly soiled
or wet, visibly damaged, or if the respirator
becomes notably harder to breathe through,
current practice shows that conservatively, the
filters could be used for at least one year.1 Although
more popular in industry settings, EHMRs have
been leveraged both before and during the
COVID-19 public health emergency and have been
highlighted in several recent media reports.2-5
Although EHMRs require a higher up-front cost
than N95 FFRs, the EHMR facepiece and cartridge
reusability may provide cost-savings advantages
and may create less hospital waste compared to
the disposable N95 FFR.6 For example,
due to COVID-19 N95 FFR shortages, one large
academic medical center—comprising 12
hospitals—purchased and deployed 10,000 EHMRs
that reduced N95 FFR usage to zero.7,8 The
center reported a significant cost benefit. The
one-time cost and storage of EHMRs was 10 times
less expensive after one month of use when
compared to disposable N95 FFRs.7,8
Research has shown that user acceptance, fit
testing, and disinfection are not barriers
to implementing EHMRs.9-12 With
proper use, fit, and maintenance training, EHMRs
provide an effective solution to supplementing
the supply of N95 FFRs.
Generally, EHMRs have exhalation valves, which
should be taken into consideration before use in
a sterile setting or for use as source control.
Until more research is available, masks with
exhalation valves or vents should NOT be worn to
help prevent the person wearing the mask from
spreading COVID-19 to others (source control).
Here are some tips when it comes to exhalation
valves:
-
Wear a respirator without an
exhalation valve when both
source control and respiratory
protection are required.
-
If only a respirator with an
exhalation valve is available
and source control is needed,
cover the exhalation valve with
a surgical mask, procedure mask,
or a cloth mask that does not
interfere with the respirator
fit.
https://blogs.cdc.gov/niosh-science-blog/2020/09/08/elastomeric/
SPG Tests Show That Flat Fold N95s Provided
Better Seal Than Cup Shaped
Twenty subjects underwent quantitative
respirator fit testing with two styles (flat
fold, cup-shaped) of N95 filtering facepiece
respirators (N95 FFRs). Passing a fit test was
followed by stereophotogrammetry to determine
the face seal area and computation of seal
pressure. There were significantly different
seal pressures (p < 0.01) between standard size
flat fold and cup-shaped N95 FFRs but no
significant differences in face seal area. No
significant differences were noted in fit
factors, but more individuals passed fit testing
wearing flat fold respirators. The ability of
flat fold N95 FFRs, at lower seal pressures, to
obtain similar fit factors as cup-shaped N95 FFR
at higher seal pressures offers the possibility
of enhanced facial comfort without a loss of
protection. Stereophotogrammetry offers a
relatively simple, non-invasive technology to
evaluate various properties of N95 FFR fit.
SPG enables the determination of geometric
properties from photographic images. This
process involves estimating the
three-dimensional (3D) coordinates of points on
an object. Photographs are taken from multiple
locations (lines of sight) and, using the
principle of triangulation (mathematical
intersection of lines of sight), the X, Y, and Z
coordinates of each point of interest are
determined.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4545596/
Eight Million Deaths Caused by Polluted Air Each
Year
New evidence on the harm caused by air
pollutants needs to be considered when
creating HVAC and mask strategies.
More than eight million people around the world
die each year as a result of breathing polluted
air that contains particles from fossil fuels, a
new study has found.
Burning fossil fuels such as coal and oil
produces greenhouse gases that trap solar
radiation in the atmosphere and cause climate
change. But it also releases tiny poisonous
particles known as PM2.5. Small enough to
penetrate deep into the lungs, these particles
can aggravate respiratory conditions like asthma
and can lead to lung cancer, coronary heart
disease, strokes and early death.
Research has also found a link between higher
levels of long-term pollution and more deaths from
Covid-19.
In a study published in the journal
Environmental Research on Tuesday,
researchers from Harvard University, in
collaboration with the University of Birmingham,
the University of Leicester and University
College London, found that exposure to
particulate matter from fossil fuel emissions
accounted for 18% of total global deaths --
almost one in five -- in 2018.
The figure is much higher than previously
thought. As recently as in 2019, scientists were
estimating that 4.2 million people die each year
from outdoor airborne particulate matter
pollution, a figure that included people who die
because of pollution from dust and smoke from
wildfires and agricultural fires.
The new study shows that in 2018, estimated 8.7
million deaths were linked to fossil fuel
emissions alone
Eloise Marais, an associate professor in
physical geography at UCL and a co-author of the
study, said the research adds to the "mounting
evidence" that air pollution from fossil fuels
is detrimental to global health.
"We can't in good conscience continue to rely on
fossil fuels, when we know that there are such
severe effects on health and viable, cleaner
alternatives," she said in a statement.
The scientists used a global 3D model of
atmospheric chemistry developed at Harvard to
get a better picture of pollution at a more
local level.
Traditionally, satellite and surface
observations were used to estimate the average
global annual concentrations of PM2.5 particles
in the air. By using the 3D model, the
scientists were able to divide the globe into a
grid with boxes as small as 50 kilometers by 60
kilometers (31 miles by 37 miles) and look at
pollution levels in each box individually.
This allowed them to assess the impact of the
pollution in places where people live and to
distinguish between different sources of
pollution.
They found that China, India, parts of the
eastern US, Europe and Southeast Asia were the
worst impacted. According to the data, as many
as 30.7% of deaths in Eastern Asia, 16.8% in
Europe and 13.1% in the US can be attributed to
fossil fuel pollution.
To model the pollution, the researchers used
real emissions and meteorology data, mostly from
2012. The year was picked to eliminate the
influence of the El Niño phenomenon, which can
worsen or improve pollution depending on the
region. They then updated the data to reflect a
44% fall in fossil fuel pollution in China
between 2012 and 2018.
The researchers estimate that China's move to
cut its fossil fuels emissions saved 2.4 million
lives worldwide, including 1.5 million in China.

